Over 2000 Fifers sign up for COVID vaccine trials
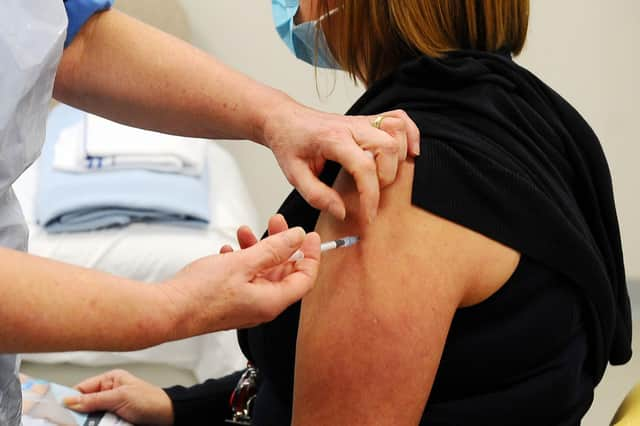

They signed up to a UK-wide appeal to take part in the clinical trials.
Researchers needed people to take part in studies to find out which potential vaccine is most effective, and those involved have to visit a hospital or research site every few months.
Advertisement
Hide AdAdvertisement
Hide AdThe largest proportion (42%) were aged between 40 and 59, while 30% were aged 60 to 79.
Across Scotland as a whole, 29,500 people signed up as the national army of volunteers reached 379,000.
£6 billion Forth rail tunnel between Kirkcaldy and Leith proposed by...Work starts on 85 new affordable homes on site of former paper mill...
Advertisement
Hide AdAdvertisement
Hide AdThe figure is rising daily, as people can still put themselves forward to potentially take part in clinical trials.
The NHS is working with the National Institute for Health Research to provide a volunteer service.
A spokesman said: "Vaccines are the most effective way to prevent infectious diseases. They are designed so they do not give people the infection they're protecting against.
"Research into vaccines is the only way to find out which ones will work."
Three vaccines have so far been approved in the UK.
Advertisement
Hide AdAdvertisement
Hide AdSpeaking about the approval of the Oxford vaccine, Professor Chris Whitty, who co-leads the NIHR, said: "The dedication and hard work of scientists, regulators and those who funded the research, such as the NIHR, United Kingdom Research and Innovation and United Kingdom Vaccine Network, and the willingness and selflessness of so many volunteers who took part in the vaccine trials were essential in delivering this safe and effective vaccine.
"They deserve our recognition and thanks.”